Image Wisely, a joint initiative of ACR, RSNA, ASRT and AAPM,
provides information to the medical community to promote safety in medical imaging.

Modern Fluoroscopy Imaging Systems
Eric Gingold, PhD, Thomas Jefferson University, Philadelphia, PA
Download PDF

Summary
Fluoroscopy, or real-time projection X-ray imaging, has been in clinical use since shortly after Roentgen’s discovery of X-rays. Early fluoroscopes consisted simply of an X-ray source and a fluorescent screen, between which the patient was placed. After passing through the patient, the remnant beam impinged upon the fluorescent screen and produced a visible glow, which was directly observed by the practitioner.
In modern systems, the fluorescent screen is coupled to an electronic device that amplifies and transforms the glowing light into a video signal suitable for presentation on an electronic display. One benefit of the modern system compared to the earlier approach is that the fluoroscopist need not stand in close proximity to the fluorescent screen in order to observe the live image. This results in a substantial reduction in radiation dose to the fluoroscopist. Patients receive less radiation dose as well, because of the amplification and overall efficiency of the imaging system.
Fluoroscopy differs from most other X-ray imaging in that the images produced appear in real-time, allowing evaluation of dynamic biological processes and guiding interventions. Electronic fluoroscopy systems create this perception by capturing and displaying images at a high frame rate, typically 25 or 30 frames per second. At these frame rates, the human visual system cannot distinguish frame-to-frame variation and motion appears to be continuous, without visible flicker. To achieve high frame rates while keeping cumulative radiation dose to a reasonable level, the radiation dose to the image receptor per image (i.e., per frame) must be kept quite low, about 0.1% of the dose used in radiography.
Fluoroscopic images appear with an inverted grayscale (black/white is reversed) compared with standard radiographs. This convention is derivative of the appearance of the early non-intensified fluoroscopic screens, and it has been retained in the digital age even though the capability now exists to digitally reverse the grayscale.
Introduction
A schematic of an image-intensified fluoroscopy system is shown in Figure 1. The key components include an X-ray tube, spectral shaping filters, a field restriction device (aka collimator), an anti-scatter grid, an image receptor, an image processing computer and a display device. Ancillary but necessary components include a high-voltage generator, a patient-support device (table or couch) and hardware to allow positioning of the X-ray source assembly and the image receptor assembly relative to the patient.
Figure 1. Schematic Diagram of a fluoroscopic system using an X-ray image intensifier (XRII) and video camera
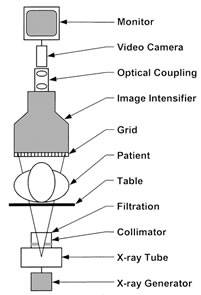
Reprinted from RadioGraphics;20(4), Schueler BA, The AAPM/RSNA physics tutorial for residents general overview of fluoroscopic imaging – Fig 2, p1117, 2000, with permission from RSNA.
X-ray Source
The high-voltage generator and X-ray tube used in most fluoroscopy systems is similar in design and construction to tubes used for general radiographic applications. For special purpose rooms such as those used for cardiovascular imaging, extra heat capacity is needed to allow angiographic “runs,” sequences of higher-dose radiographic images acquired in rapid succession to visualize opacified vessels. These runs are often interspersed with fluoroscopic imaging in a diagnostic or interventional procedure, and the combination can result in a high demand on the X-ray tube. Special X-ray tubes are generally found in such systems.
Focal spot sizes in fluoroscopic tubes can be as small as 0.3 mm (when high spatial resolution is required but low radiation output can be tolerated) and as large as 1.0 or 1.2 mm when higher power is needed. The radiation output can be either continuous or pulsed, with pulsed being more common in modern systems. Automatic exposure rate control maintains the radiation dose per frame at a predetermined level, adapting to the attenuation characteristics of the patient’s anatomy and maintaining a consistent level of image quality throughout the examination.
Beam Filtration
It is common for fluoroscopic imaging systems to be equipped with beam hardening filters between the X-ray tube exit port and the collimator. Added aluminum and/or copper filtration can reduce skin dose at the patient’s entrance surface, while a low kVp produces a spectral shape that is well-matched to the barium or iodine k-edge for high contrast in the anatomy of interest.
Insertion of this added filtration in the beam path may be user-selectable, providing the operator with the flexibility to switch between low dose and higher dose modes as conditions dictate during a fluoroscopic procedure. In other systems the added filtration is automatic, based on beam attenuation conditions, to achieve a desired level of image quality and dose savings.
In addition to beam shaping filters, many fluoroscopy systems have “wedge” filters that are partially transparent to the X-ray beam. These moveable filters attenuate the beam in regions selected by the operator to reduce entrance dose and excessive image brightness.
Collimation
Shutters that limit the geometric extent of the X-ray field are present in all X-ray equipment. In fluoroscopy, the collimation may be circular or rectangular in shape, matching the shape of the image receptor.
When the operator selects a field of view, the collimator blade positions automatically move under motor control to be just a bit larger than the visible field. When the source-to-image distance (SID) changes, the collimator blades adjust to maintain the field of view and minimize “spillover” radiation outside of the visible area. This automatic collimation exists in both circular and rectangular field of view systems.
Patient Table and Pad
Patient tables must provide strength to support patients and are rated by the manufacturer for a particular weight limit. It is important that the table not absorb much radiation to avoid shadows, loss of signal and loss of contrast in the image.
Carbon fiber technology offers a good combination of high strength and minimal radiation absorption, making it an ideal table material. Foam pads are often placed between the patient and the table for added comfort, yet with minimal radiation absorption.
Anti-Scatter Grid
Anti-scatter grids are standard components in fluoroscopic systems, since a large percentage of fluoroscopic examinations are performed in high-scatter conditions, such as in the abdominal region. Typical grid ratios range from 6:1 to 10:1. Grids may be circular (XRII systems) or rectangular (FPD systems) and are often removable by the operator.
Image Receptor — X-ray Image Intensifier (XRII)
The X-ray image intensifier (Figure 2) is an electronic device that converts the X-ray beam intensity pattern (aka, the “remnant beam”) into a visible image suitable for capture by a video camera and displayed on a video display monitor. The key components of an XRII are an input phosphor layer, a photocathode, electron optics and an output phosphor.
The cesium iodide (CsI) input phosphor coverts the X-ray image into a visible light image, much like the original fluoroscope. The photocathode is placed in close proximity to the input phosphor, and it releases electrons in direct proportion to the visible light from the input phosphor that is incident on its surface. The electrons are steered, accelerated and multiplied in number by the electron optic components, and finally impinge upon a surface coated with a phosphor material that glows visibly when struck by high-energy electrons. This is the output phosphor of the XRII.
In principle, one could directly observe the intensified image on the small (1” diameter) output phosphor, but in practice a video camera is optically coupled to this phosphor screen through an adjustable aperture and lens. The video signal is then displayed directly (or digitized), post-processed in a computer and rendered for display.
Figure 2. Components of an X-ray image intensifier
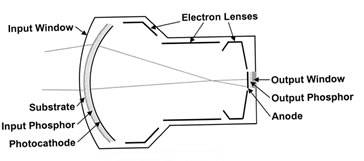
Reprinted from RadioGraphics;20(4), Schueler BA, The AAPM/RSNA physics tutorial for residents general overview of fluoroscopic imaging – Fig 5, p1120, 2000, with permission from RSNA.
The XRII achieves orders of magnitude more light per X-ray photon than a simple fluorescent screen. This occurs through electronic gain (amplification by the electron optics) and minification gain (concentrating the information from a large input surface area to a small output phosphor area) as shown in Figure 2. This allows relatively high image quality (signal-to-noise ratio) at modest dose levels compared with non-intensified fluoroscopy.
The use of video technology added an important convenience factor — it allows several people to observe the image simultaneously and offers the ability to record and post-process fluoroscopic image sequences.
Image intensifiers are available in a variety of input diameters, ranging from about 10–15 cm up to 40 cm. The input surface is always circular and curved, a design characteristic of the vacuum tube technology from which it is constructed.
The video cameras used in XRII systems were originally vidicon or plumbicon analog devices borrowed from the broadcast television industry. In later systems, digital cameras based on charge-coupled device (CCD) image sensors or complementary metal oxide semiconductor (CMOS) technology came into common use.
Image Receptor — Flat Panel Detector (FPD)
In recent years we have seen the introduction of fluoroscopic systems in which the XRII and video camera components are replaced by a “flat panel detector” (FPD) assembly. When flat panel X-ray detectors first appeared in radiography, they offered the advantages of a “digital camera” compared with existing technologies.
In fluoroscopic applications, the challenge for FPDs has been the requirement of low dose per image frame, meaning that the inherent electronic noise of the detector must be extremely low, and the required dynamic range is high. It has proven to be quite difficult to manufacture FPDs with electronic noise characteristics low enough to achieve good signal-to-noise ratio (SNR) under low exposure conditions, yet such devices do now exist.
Flat panel detectors are more physically compact than XRII/video systems, allowing more flexibility in movement and patient positioning. However, the most important benefit of the FPD is that it does not suffer from the many inherent limitations of the XRII, including geometric “pin-cushion” distortion, “S” distortion, veiling glare (glare extending from very bright areas) and vignetting (loss of brightness at periphery). These phenomena simply do not occur in FPDs. FPDs often have wider dynamic range than some XRII/video systems.
Another advantage of FPDs is that the image receptor’s spatial resolution is defined primarily by the detector element size, and unlike the XRII/video, is independent of the field of view. In XRII systems, the minification gain requires the entrance dose to vary inversely with field-of-view to maintain a constant brightness at the output phosphor. No such constraint exists for FPDs; the entrance detector dose is independent of the field of view.
Flat panel detectors consist of an array of individual detector elements. The elements are square, 140–200 microns per side and are fabricated using amorphous silicon thin-film technology onto glass substrates.
Detector arrays used for fluoroscopy range from about 20 x 20 cm up to 40 x 30 cm. A single detector may contain as many as 5 million individual detector elements. A cesium iodide (CsI) scintillation layer is coated onto the amorphous silicon, with thin-film photodiodes and transistors capturing the visible light signal from the scintillator to form the digital image, which is then transferred to a computer at a frame rate selected by the user (Figure 3). Frame rates can be as high as 30 frames per second.
Figure 3. Cross-section of flat panel detector for fluoroscopic imaging
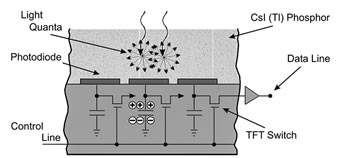
Reprinted from Radiology; 234(2), Pisano ED, Yaffe MJ, State of the Art: Digital Mammography – Fig 1, p355, 2005, with permission from RSNA.
Image Display
Fluoroscopy requires high-quality video displays that allow users to appreciate fine details and subtle contrast differences in the anatomy of interest. Medical image display technology has been fortunate to “ride on the coattails” of the television industry over the last several years.
Modern systems feature high resolution flat-panel LCDs with high maximum luminance and high-contrast ratios. These displays should be calibrated to a standard luminance response function (such as the DICOM part 14 Grayscale Standard Display Function) to ensure that the widest range of gray levels are visible.
The newest interventional/angiographic systems feature 60” diagonal high-definition displays supporting up to 24 different video input sources that can be arranged in various ways on the single large display monitor. Display layouts can be uniquely customized and saved for individual physician preference.
System Configurations
Fluoroscopic systems are manufactured in a variety of configurations to optimize usability for the clinical task(s) for which they are intended. “Conventional” radiography/fluoroscopy systems consist of a patient table that often tilts all the way to vertical position permitting fluoroscopy while the patient stands upright. These systems have the X-ray tube positioned under the table-top, with the image receptor above the table, and are most often used for gastrointestinal imaging (upper and lower GI barium enhanced studies).
The tilting capability of the patient table allows the operator to utilize gravity to assist the movement of the barium contrast material through the esophagus, stomach and bowel. Older systems may contain a “spot film” device that allows placement of a radiographic cassette in front of the fluoroscopic image receptor, facilitating the acquisition of radiographs using the fluoroscopic X-ray source. In modern systems, static images are routinely acquired using the same digital image receptor that is used for fluoroscopy, so the spot film is going away.
A variation on this conventional R/F configuration is the remote controlled system, in which the X-ray tube and image receptor positions are reversed with the tube above the patient table and the image receptor below. These systems can be fully controlled, including table movements, at an operator’s console featuring a joystick-type controller in a shielded control booth. This protects the staff from secondary radiation exposure.
Angiographic systems employ a “C-arm” geometry to enable easy patient access as fluoroscopy guides selective arterial and venous catheter placement. These systems include advanced features like digital subtraction and road mapping.
The newest systems have 3D imaging capability, achieved by spinning the C-arm around the patient and performing a tomographic reconstruction to produce a volumetric image data set. This is sometimes referred to as cone beam CT (CBCT), while in angiographic mode it is known as 3D rotational angiography. Systems designed for vascular/interventional radiology and cardiology/electrophysiology have sophisticated fluoroscopic capabilities, including variable frame rate, automatic beam filtration and advanced image post-processing. Finally, the mobile C-arm configuration is popular in the surgical suite and for office-based procedures in musculoskeletal radiology, orthopedics, urology, gastroenterology and pain management among others. Mobile C-arms are often small inexpensive systems, but some are available with higher-power X-ray sources that have the ability to produce substantial radiation output levels.
Summary
Fluoroscopy has evolved from the most simplistic of non-invasive imaging methods to a very sophisticated technology with advanced 3-D capabilities, able to guide life-saving interventional procedures often with minimal patient discomfort. Many of these minimally invasive image-guided procedures have replaced highly invasive open surgical procedures. With each incremental advancement in the technology, smaller vessels and more subtle contrast differences can be visualized in real time, often with low radiation dose.
References
- Schueler BA. The AAPM/RSNA physics tutorial for residents general overview of fluoroscopic imaging. RadioGraphics, 2000. 20(4):p1115-1126. Available at: http://pubs.rsna.org/doi/full/10.1148/radiographics.20.4.g00jl301115. Accessed October 23, 2014.
- Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The Essential Physics of Medical Imaging. Philadelphia, PA, Lippincott Williams & Wilkins; 3rd ed, 2012. Available at: http://books.google.com/books?id=RKcTgTqeniwC&printsec=frontcover&dq=The+Essential+Physics+of+Medical+Imaging,+3rd+Edition&hl=en&sa=X&ei=L-tIVLbCIs6zyASEioK4Bw&ved=0CDIQ6AEwAA#v=onepage&q=The Essential Physics of Medical Imaging, 3rd Edition&f=false. Accessed October 23, 2014.
- Nickoloff EL. Physics of Flat Panel Fluoroscopy Systems. RadioGraphics, 2011. 31(2):p591-602. Available at: http://pubs.rsna.org/doi/pdf/10.1148/rg.312105185. Accessed October 23, 2014.
- Pisano ED, Yaffe MJ. State of the Art: Digital Mammography. Radiology, 2005. 234(2):p353-362. Available at: http://pubs.rsna.org/doi/full/10.1148/radiol.2342030897. Accessed October 23, 2014.



